stem cell treatment for rheumatoid arthritis
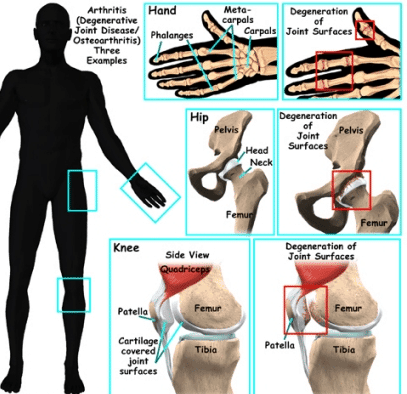 Stem Cell Therapy for Arthritis - Rheumatoid & Osteoarthritis
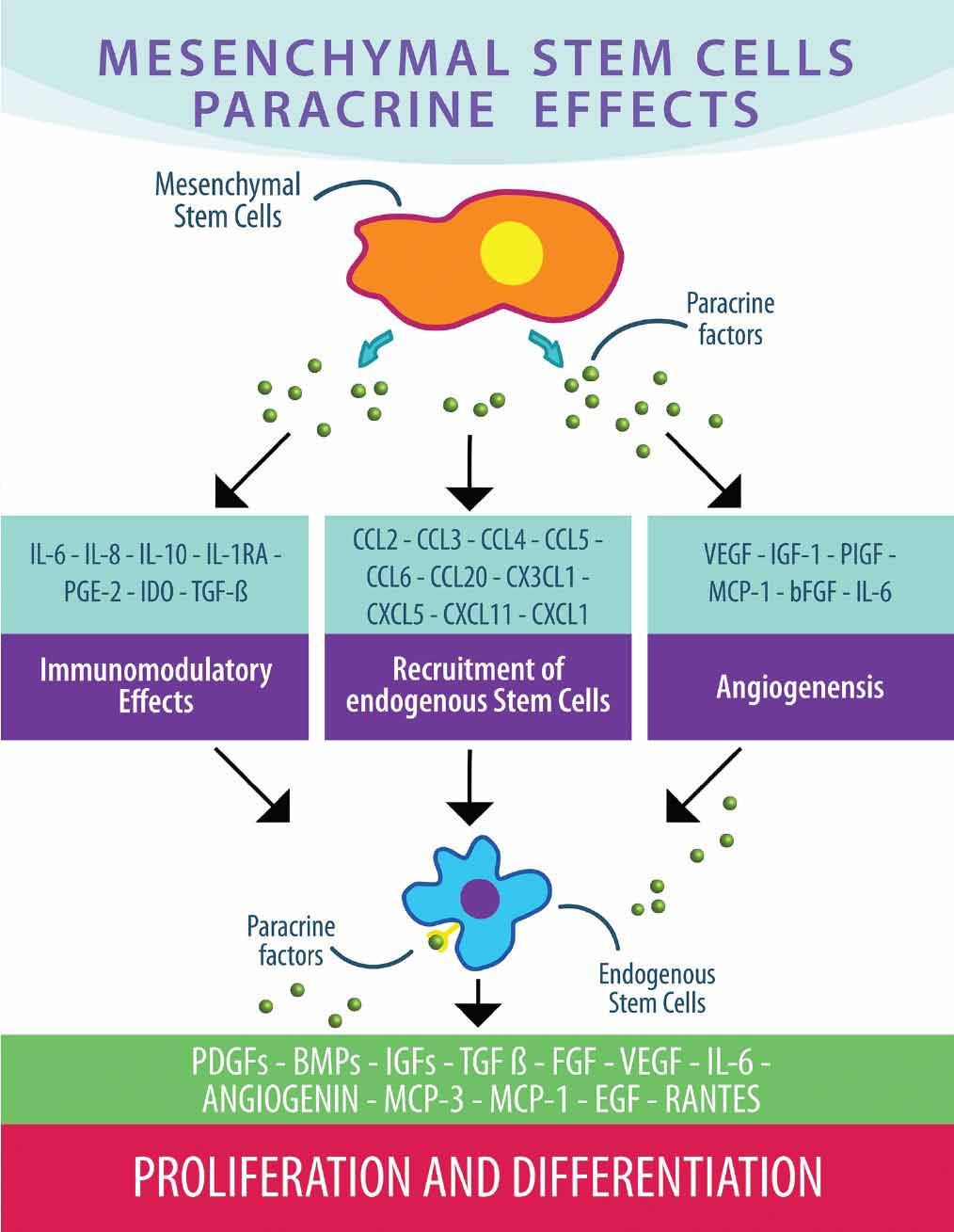
Stem Cell Therapy for Arthritis - Rheumatoid & OsteoarthritisWarning: The NCBI website requires JavaScript to operate. Mesenchymal stem cells Improve rheumatoid arthritis Progression for Controlling Memory T Cellular ResponseNoymar Luque-Campos1Culular and Molecular Immunology Laboratory, Biomedical Research Center, Faculty of Medicine, Universidad de los Andes, Santiago, ChileRafael A. Contreras-López1Laboratorio de Inmunología Celular y Medicina Centro María Jose Paredes-Martínez1Laboratorio de Inmunología Celular y Molecular, Centro de Investigación Biomédica, Facultad de Medicina, Universidad de los Andes, Santiago, Chile Maria Jose Torres2Escuela de Ingeniería Bioquímica, Pontificia Universidad Católica de Valparaíso, Valparaísox In recent years, mesenchymal stem cell (MSC)-based therapies have become an interesting therapeutic opportunity for the treatment of rheumatoid arthritis (RA) due to its ability to powerfully modulate the immune response. RA is a chronic autoimmune inflammatory disorder with an incompletely understood etiology. However, it has been well described that peripheral tolerance defects and the subsequent abnormal infiltration and activation of various immune cells in the synovial membrane are critical to the development and progression of the RA. In addition, the imbalance between the immune response of pro-inflammatory and anti-inflammatory cells, in particular between Th17 memory and T-regulatory memory cells (Treg), respectively, is well admitted that it is associated with RA immunopathogenesis. In this context, MSCs, which are able to alter the frequency and function of memory lymphocytes including Th17, follicular aid cells T (Tfh) and gamma delta (γδ) T cells by promoting the generation of Treg cells, have been proposed as a candidate for choice for RA cell therapy. In fact, given the plasticity of the CD4+ Cells T memory, it is reasonable to think that MSCs will restore the balance between T cells populations of pro-inflammatory and anti-inflammatory memory deregulated in the RA that leads to boost their therapeutic function. In the present test, we will discuss the role of the memory T cells involved in RA pathogenesis and the beneficial effects exerted by MSCs on the phenotype and functions of these abnormally regulated immune cells in the RA and how this regulation could affect the progression of the RA. Introduction Mesenchymal stem cells (MSC) are multi-potent stem cells capable of performing immunosuppressive functions in both innate cells and adaptive immune cells (). They have been isolated from almost all the mesoderal tissues including bone marrow, adipose tissue, umbilical cord blood, umbilical cord, placenta, menstrual fluid and dental pulp (–). The International Society of Cell Therapy (ISCT) has defined minimum criteria to characterize MSCs that include a morphology similar to fibroblastics, the expression of mesodermic markers such as CD90, CD105, and CD73, the lack of expression of hematopoietic markers such as CD45, CD34, CD14, and the ability to differentiate in adipocytes, condrocytes and osteoblates. MSCs have been reported as an interesting candidate of therapeutic cells for the treatment of autoimmune diseases such as the RA, due to their ability to mitigate the exacerbated pathogenic immune response observed in these patients (). However, given the complexity of RA disease, as well as the mechanisms involved in MSC immunosuppressive functions, it is mandatory to decipher the mechanism by which MSC mediated its immunosuppressant potential in the subsets of immune cells associated with RA to improve MSC-based therapy. In this context, one of the main objectives for MSC-based therapy are pathogenic T-cells due to their critical role in the progression of autoimmune disease including RA (). There is currently no article that focuses on discussing the importance of selecting T cells with MSC-based therapy for the treatment of autoimmune diseases. Therefore, in this review, we will focus on the effect of MSCs on CD4+ memory T cells subset and discuss the advantage that this knowledge could give to improve their immunosuppressive properties in order to develop novel MSCs-based therapy for RA treatment. During the development of this review, we will discuss the role of T memory cells in the evolution of RA-centric autoimmune disease and will infer studies between MSCs and their impact on T memory cells and how the regulation of this population could be a key player in improving the RA. MSC-based therapy for the treatment of autoimmune diseases MSCs have been proposed to a large extent as a therapeutic tool for the treatment of autoimmune diseases due to their powerful suppressive activity to inhibit pro-inflammatory cells from both the innate system and the adaptive immune system. In fact, it has been reported that MSCs are able to modulate the differentiation and function of myeloid cells towards immunosuppressant phenotypes. These cells include monocytes (, ), dendrite cells (DCs) (, ), macrophages (), myeloid suppressant cells (MDSCs) (), and neutrophils (). In addition, MSCs inhibit the proliferation of T cells (, ) and B cells (), as well as their functions. Mechanisms involved in this immunomodulation include cell contact and the production of soluble factors (). In addition, MSCs are able to migrate to inflammatory sites to interact and modulate pro-inflammatory immune cells at the site of inflammation (). For all these reasons, we can currently have a total of 707 clinical trials related to MSCs registered in the NIH clinical trial database (). These clinical trials mainly tend to evaluate the therapeutic effectiveness and safety of MSCs from different sources. In addition, until December 2018 there are several clinical trials aimed at treating autoimmune diseases such as multiple sclerosis (n = 29), Crohn's disease (n = 7), systemic lupus erythematosus (SLE) (n = 12), and RA (n = 14). In general, the short- and long-term use of MSC-based therapy gives positive effects without reporting severe adverse events in addition to some immediate type I hypersensitivity (pruritis, rash, fever) in However, despite these results there is still much controversy about the positive effects of MSC-based therapy as its effect strongly depends on the etiology of the disease and the degree of inflammation. It is therefore very important to understand the interaction between MSCs and pathogenic immune cells such as T memory cells, as they are the main players in the generation, pathogenesis and progression of autoimmune disease. Memory Cells T: Key Player in the pathogenesis of autoimmune disease After infection or immunization, naive T cells experience a clonal expansion that leads to a high frequency of T cells specific to the antigen with a rapid effect function. Naïve CD4+ T cells can differentiate in multiple subsets of T-efector T cells (Th) such as Th1, Th2, Th17 and T-equipment helper cells (Tfh) among others, while CD8+ ingenuo T cells are differentiated in cytotoxic T lymphocytes (TCL) (). Once the initial response of the adaptive immune system against an antigen ends, the organism must return to homeostasis through the contraction of T cells from the effectr. During this period the small amount of cells that survive will eventually become part of the immune memory: immune cells that are able to respond quickly to a second round of a specific antigen previously found (). The generation and persistence of T memory cells is an important feature of the adaptive immune system acquired after exposure to antigen that provides permanent protection against infections (). The T memory cells are a heterogeneous population of cells classicly distinguished by the expression of the CD45RO isoform and by the absence of CD45RA (CD45RO+CD45RA−) (, ). Lately, in specific human subsets of CD4+ and CD8+ memory T cells were identified in peripheral mononuclear cells (PBMCs) through the expression of receptor 7 (CCR7), a chemotherapy receptor that controls the homing to secondary lymphoid organs (). The CCR7 negative memory T cells were found to produce more effective cytokines, compared to the CCR7 positive subset (). Based on this finding, two subsets of memory T cells were identified: CCR7+ central memory T cells (MCCs) and CCR7 - T-remembrance cells of the effectr (TEM) (). Several studies have been conducted to characterize the memory cells present in PBMC using an extensive marker panel. CD44hi, CD45ROhi, CD45RAlow, CD127hi, CD62LhiCCR7hi TCM cells are generated and resided in secondary lymphoid tissues in the absence of antigen, while CD44hi, CD45ROhi, CD45RAlow, CD127hi, L-selectinlow CCR7low TEM cells, are generated in secondary lymphoid tissues and are recycled between blood and non-lyphoid tissues in the absence of antigen (). As mentioned above, long-lived memory T cells in the presence of secondary antigen exposure expand and develop a stronger and stronger response. In the case of autoimmune diseases, T cells can become harmful against autoantigens as these memory cells present a powerful pathogenic response against auto-sues. In addition, due to their longevity, they are very difficult to eliminate, so the development of new therapies directed against these cells is of fundamental importance to control autoimmunity. In this context, the role of T cells in memory in autoimmune diseases has been studied. Patients with MS have a high number of T cells (–), especially TEM subsets (, ). Recently, CD4+ memory has been reported CCR9+T cells are altered in MS patients and may mediate the development of progressive secondary MS progression (). It has also been reported that the subpopulation of T-memory cells is increased in active Crohn-s disease patients (, ). In fact, peripheral blood and intestinal mucous memory Crohn's T cells have increased intracellular production of TNFα and correlate with the disease score (CDAI). In addition, this peripheral memory of TNFα blood produces TNFα cells have an increased migratory profile to extra nodal lymphoid tissues such as intestinal mucosa (). In addition, there are tests that suggest an increase in the population of CD4+ TEM cells in the SLE pathogenesis (). In addition, PD1+ICOS+TCM and PD1+ICOS+TEM subpopulation increase in patients with SLE and TEM cells positively correlative with the severity of the disease (). In addition, an enrichment of genes associated with CD4+ TEM cells has been observed within SLE loci, Crohn loci and loci RA (). All these evidences point to the memory of T cell subsets as the main contributors of autoimmune pathogenicity. The role of T-memorial cells in RARA development and progression is an autoimmune disease characterized by the high production of antibodies that affect a wide variety of autoantigens. Among them, rheumatoid factor (RF) and anti-citrullin protein antibodies (ACPA) have been the most described (). RA immunopathogenesis is characterized by deficiencies in the immune response with predominance of pro-inflammatory cells and an alteration of peripheral immune tolerance that involves in particular CD4+ T cells (, ). CD4+ T cells of RA patients go through a premature transition from a naïve to a phenotype of memory. The resulting memory CD4+ T cells are hyperproliferative due to cell cycle control failures that promote their differentiation to Th1 and Th17 pathogenic T cells (). This was confirmed in studies that show that RA patients have a large number of memory CD4 T cells that infiltrate the swollen synovial membrane (–). In addition, a higher frequency of subset of TEM cells was observed in the synovial fluid of patients with RA (). While TEM cells have a short life, they have a powerful effector function with a high capacity to secrete pro-inflammatory cytokines that allow them to respond faster to the antigens present in the synovial fluid (). All together, these studies suggest the presence of highly activated and differentiated memory CD4+ Cells T with a high capacity to produce pro-inflammatory cytokines in synovial fluid of patients with RA. Conventional therapy for the treatment of RA There is currently a wide variety of medications aimed at reducing symptoms and gradual progression of the disease. Among them, synthetic anti-rheumatic drugs (SDMARD) that modify synthetic disease (MTX), leflunomide, sulfasalazine and hydroxychloroquine, biological response modifiers called biologics (bDMARD) and corticosteroids. All these treatments aim at inflammation and are aimed at improving the quality of life and prognosis of RA patients () through the prevention of structural damage (erosive disease) and control of extra-articular symptoms. Since then, the pathogenesis of the RA is associated with alterations of immune cell functions and secretion of cytokines produced in part by CD4+ pro-inflammatory.Memory response T cells have been proposed, a wide variety of BDMARDs to target these latest cells. For example, BDMARD's first test was aimed at reducing the production of alpha tumor necrosis factor (TNF-α) (Infliximab), a pro-inflammatory cytokine highly produced by RA patients' T-cell memory. Since then, other TNF agents such as etanercept, adalimumab, certolizumab and golimumab, as well as other biological agents such as anti-IL6 (tocilizumab), anti-CTLA4 (abatacept), and anti-CD20 (Rituximab) were developed. However, the treatment of some patients with TNF inhibitors did not significantly reduce the rate of Th17 pathogenic cells by revealing that a high range of patients do not respond to this treatment (). Later, an anti-interleukin antibody 17 (IL-17) (secukinumab) and anti-IL-17RA brodalumab antibody (AMG827) was developed and evaluated in clinical trials, including RA patients with an inadequate response to metotrexate. The phase II clinical study on RA patients showed that the brodalumab administration did not improve the progression of the RA as revealed by the minimum response criteria established by the American College of Rheumatology (ACR) (). Similar results were observed after the administration of secukinumab in a phase Ib clinical trial that included moderate to severe RA patients (). In fact, the administration of these drugs did not reduce the frequency of memory of Th17 cells. Curiously, RA patients treated with TNF inhibitors, have Th17 pathogenic cells with a blurred phenotype due to the high production of granulocyte-macrophagus colony stimulation factor (GM-CSF) (). In fact, GM-CSF is indispensable for the differentiation of inflammatory dendrite cells (infDCs) that induce the activation of CD4+ T cells that produce IL-17 (, ). Thus, a monoclonal antibody against GM-CSF has been developed and has been described effectively in the clinical trial for the treatment of RA (). However, despite this promising result, the use of anti-GM-CSF antibody has not yet been approved (). Canine inhibitors such as Tofacitinib and Baricitinib have also been developed for the treatment of RA (, ). These inhibitors block the activation of signal transducer and transcription signaling pathway activator (STAT), which drive the signature of many cytokines including interleukin-7 (IL-7) and interleukin-15 (IL-15) that are important for the proliferation and survival of T memory cells (–). Another approach was the development of drugs that imitate mechanisms produced naturally by our own immune system. For example, Abatacept is a soluble recombinant human fusion protein that includes the extracellular domain of human cytotoxic antigen T-Lymphocyte 4 (CTLA-4). This protein binds to CD80 and CD86 receptors in cells that represent antigen (APC) and blocks interaction with T cells through the co-stimulation molecule CD28 (). Clinical trials have shown promising results using RA Treatment Abatacept (). However, a CD4+ subset of tissue infiltration It has been shown that the T cells of a group of RA patients lose the expression of CD28 while they begin to express memory markers (, ). These latest cells exhibit high capacity to produce pro-inflammatory cytokines such as interferon-gamma (IFNγ) and TNFα and cytotoxic activity (–). It is worth noting that the effect of BDMARD administration has never been addressed in the population of T cells of memory. Although significant progress has been made with the current state RA treatment to obtain long-term induction, between 20 and 30% of patients with moderate to constant RL do not respond positively to mono or combination therapy (more Methotrexate) with these agents () therefore, the development of new therapies directed to pathogenic memory cells T seems to be ideal for improving RA progression. MSC-based therapy for RA treatment Although MSC-based therapy for RA treatment is one of the main models of autoimmune disease to study the mechanism underlying the therapeutic effect of MSCs, today, MSC-based clinical trials have been the least studied in autoimmune diseases. In this context, there are 14 clinical trials of MSC-based therapy for RA. In them, it has been reported that the intravenous infusion of alogenic bone marrow and umbilical cord MSC in a small group of refractory RA patients resistant to antibodies anti-TNF monoclonal antibodies, led to a reduction in the sedimentation rate of erythrocytes, improved in the clinical score DAS28 and decreased in the level of temporary anticyclic cytruline only. In another study, using alogenic UC-MSCs for RA treatment, safety and effectiveness was demonstrated in a greater number of patients (). In this study, MSCs and DMARDs were administered intravenously in 172 patients with active RA inducing a significant increase in the percentage of CD4+ regulated Cells T (Treg) in the blood along with a significant clinical improvement of up to 6 months. In addition, the repeated infusion of MSCs after this period allowed an increase in the therapeutic efficacy of cells (). More recently, in a phase Ib/IIa clinical trial, it was shown that the intravenous administration of adipous expanded adipous stem cells (ASCs) in a study that included 53 patients with a placebo group were safe and well tolerated in refractory RA patients (). Unfortunately at present there is no report showing immunity surveillance of RA patients after the infusion of MSCs that could allow us to compare the immune profile of RA patients treated or not with MSCs with their clinical score before and after MSCs infusion. In fact, it is obligatory to delve into how MSCs affect the pro-inflammatory cells that are detangled in these patients in particular T pathogenic memory cells. This information will surely help us understand the mechanism by which MSCs exercise their therapeutic function that will allow us to improve MSC-based therapy. MSCs immunomodulatory function in Memory T cells: Focus on RADespite the significant progress that has been made in the generation of new therapies against the RA, there are still many patients who do not respond to any treatment. It is therefore reasonable to think that the resistance of T cells of pathogenic memory could be the main contributor to the absence of a beneficial effect of these immunomodulatory therapies (, ). Therefore, it is obligatory for the successful development of RA therapies to aim these specific T cell subsets. In this context, the effect of MSCs on memory T cells has been investigated. For example, Pianta et al. demonstrated that the conditioned medium derived from the mesenchymal layer of the human amniotic membrane (CM-hAMSC) strongly inhibits the central memory (CD45RO+ CD62L+) as well as the memory of the effects (CD45RO+ CD62L−) Subsets of T cells, although later to lower degree (). In addition, using mononuclear cells of peripheral blood (PBMC) activated with phytoohemagglutinin (PHA), it has been shown that MSCs highly inhibit the proliferation of TCM, TEM and CD4+ T (D) cells. In addition, Mareschi et al. observed that MSCs derived from different tissues such as bone marrow and placenta were able to decrease the proliferation of T cells of memory (CD4+CD45RO+) (). In particular, PBMC stimulated PHA was shown to significantly decrease the frequency of CD4+ TCM and TEM cells, which produce TNF-α, IL-2 and IFNγ, when co-cultivated with BM-MSCs (). Therefore, all these studies aim to evaluate the inhibitive capacity of MSCs in human memory CD4+ T cells show a stronger immunomodulatory effect in the TCM cell subset. However, the effect exerted by MSCs on the subpopulations of memory T cells described to play a key role in RA immunopathogenesis, such as memory Th17 cells, Treg memory cells and Tfh memory cells among others still need to be investigated. The effect of MSCs on T cells of memory of particular subpopulations that could be related to RA immunopathogenesis will then be described. Effects of MSCs in Memoir Vγ9Vδ2 T Cells A high frequency of the memory of the Vγ9Vδ2 T-fector has been found in the peripheral blood and the synovial fluid of RA patients. These cells have a powerful ability to secrete inflammatory factors, such as IFNγ and IL-17, and to present antigens (). MSCs shows a powerful ability to suppress the proliferation of γδ T cell, as well as its cytolytic responses and cytokine production (, ). The latter effect is mediated by the release of MSCs from the COX-2 production of prostaglandin E2 (PGE2) through its receptors, EP2 and EP4, expressed in T Vγ9Vδ2 (, ) cells. These results suggest that MSCs exert a beneficial effect on the RA through their ability to prevent immune response dysfunction mediated by T γδ cells through inhibition of inflammatory cytokine production and improvement of anti-inflammatory response. Interaction between pro-inflammatory Memory Cells and MSCThe production of autoantibodies by B cells and therefore the production of autoantibodies in RA patients implies in part the cooperation of Tfh cells (). An association between a greater percentage of ICOS+ blood memory cells, RA patient sera antibody thyter and RA activity and/or severity (, ). The differentiation of ingenuous CD4+T cells isolated from RA patients in Tfh cells was suppressed by human UC-MSC in part through indolemine 2,3-dioxygenase (ID) activity of MSC induced by IFNγ produced by Tfh cells (). In the collagen-induced arthritis model (CIA), MSC injection prevented the progression of arthritis in mice by altering both the number and function of Tfh cells (). These results indicate that MSCs can inhibit the differentiation of Tfh to the different subsets of memory such as Tfh1, Tfh2, and Tfh17 and, therefore, decrease the number of automatic B reactive cell and the production of auto antibodies such as anti-CCP. Effects of MSC in T-memorial pro-inflammatory cellsInteractions between the chemoquinas and their respective receptors are key mediators of inflammation as they govern the accumulation and homage of CD4+ Cells T memory in the synovial membrane of RA patients. Chemokine ligand 3 (CCL3), CCL4, and CCL5 chemokines, which are highly produced by different types of cells present in the synovial tissue, are joined to several chemokin receptors such as CCR5 expressed on the surface of the memory T cells that are (, ). The CCR5 expression is increased on the surface of synovial tissue and T cells fluid and correlated with the IFN-γ expression by synovial memory CD4+ T cells of RA patients (–). Synoptic memory CD4+ T cells also express lymphoxyoxine-alpha (LT-α) that correlates with the expression CCR6 and the presence of lymphocytic aggregates in the synovial tissue (). It was proposed that CCR6 play a role in the development of CD4+ Cells T aggregates that are characteristically found in the swollen rheumatoid synovium (). As mentioned above, IL-17 plays a critical role in the inflammatory process of the RA. IL-17 improves the production of chemotherapists such as CCL20 and the entromal-derivated factor 1 (SDF-1) by the synocytes, thus promoting the recruitment of T-memorial cells to the synoxy (–). One of the mechanisms associated with the therapeutic effect of MSCs is its ability to migrate and house in inflated tissues (). The MSCs are well described to secrete constitutively a variety of different chemists such as CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL7 (MCP-3), CCL20 (MIP-3α), CCL26 (eotaxin-3), CXCL1 (GROα), CXCL8 Therefore, such MSCs could potentially migrate into the inflated synovium and interact with T memory cells, inhibit their proliferation rate or/and alter their pro-inflammatory phenotype and eventually reduce inflammation in the synovial membrane. CXCR4 plays a central role in the homing and retention of CD4+ T cells (, ). Curiously, patients with more susceptible HLA-DR heplotypes showed a significantly greater frequency of CXCR4+CD4+T memory, suggesting that synovial migration and the retention of CXCR4+CD4+ T cells are associated with sustained autoimmunity and local inflammation. In addition, the high frequency of CXCR4+CD4+ T-cells correlated with the high level of expression of HLA-DR in the underlying B cells that B-cells are important antigeno-presentative cells in the RA (). Xie et al. have reported that MSCs exhibit an increased CXCR4 expression level when Notch signaling pathway was inhibited suggesting that notch signaling regulates migration and MSC function (). Together, these studies suggest that blocking the Notch pathway could improve the MSC therapeutic effect by increasing its ability to migrate and lodge in the synvice where they will interact with the CXCR4+CD4+ T memory cells and control the RA pathogenesis. Effects of MSCs in Th17 and Treg Memory T Cells Th17 cells express the retinoic acid nuclear hormone receptor C (RORC) and secrete IL-17A along with other cytokines, including IL-17F, IL-21 and IL-22. Th17 cells are pro-inflammatory help cells that protect the body from extracellular pathogens, including gram-negative bacteria, micobacteria and fungi (). However, its deregulation is associated with the generation of autoimmune diseases including the RA (). On the other hand, it is well known that human Treg cells play a central role in maintaining immunocyte homeostasis and auto-tolerance (immunologic). Treg cells exert powerful immunosuppressive effects on the proliferation of T cells and the production of cytokines through a cytokine-dependent mechanism that requires cellular contact to cell. Treg cells are characterized by the high level of expression of CD25 (also called CD25 bright cells) and more specifically, the intracellular expression of the FoxP3 transcription factor (, ). In addition, Treg is characterized by a low expression of CD127 (IL-7 alpha-chain receptor) (), and a low regulation of CD127 that is associated with the acquisition of regulatory function (). The imbalance between the Th17 and Treg cells has been largely associated with RA pathogenesis due to its narrow differentiation pathways but its completely opposite function. (, ) In fact, Th17 cells are involved in the development of RA and progression and the high levels of IL-17 have been reported in the synovial fluid of RA patients that is positively correlated with the severity of the disease (–). In addition, the IL-17 is mainly produced by CD4+CD45RO+ memory T cell (, ). Another molecule, the CCR6 chemokin receptor, is expressed by the memory Th17 cells and is associated with its ability to migrate towards inflammatory joints in response to CCL20 highly produced by T cells and the synovites (, ). On the other hand, CD4+CD25high Treg cells are predominantly memory cells in the synovial fluid that are enriched with CD4+CD25+CD127l°wFoxP3+ treg cells in the synovial fluid of RA patients (, , ). In addition, while the percentage of memory of the subset Treg cells increased significantly in the synovial fluid of the patients of the disease, it did not change and the frequency of Treg-related blood However, despite the increase of Treg in the synovial fluid, the inflammation remains suggesting an alteration of its functions in patients with RR. This was confirmed by a body of studies that has demonstrated by the reduced regulatory functions of Treg derived from peripheral blood (–) and the synovial fluid of patients of the RA (). In line with these studies, the isolated Treg cells of patients with active RA did not inhibit the secretion of pro-inflammatory cytokines such as IFN-γ and TNFα released by T-Efector cells (–, ). Notoriously, TNFα may inhibit Treg's suppressive function () suggesting that the synovial fluid of the RA enriched in pro-inflammatory conversion memory Treg cells into cells producing pro-flammatory cytokines such as IL-17 unable to exercise regulatory functions (). Increased percentage of CD45RA memory−Foxp3low non-regulatory T cells were reported in the synovial fluid of the RA, while it did not change in the peripheral blood of patients (). Non-Treg memory cells produce IL-2, IFN-γ, and IL-17 and express high levels of RORC (, ). MSCs are powerful CD4+T-bet+CD183+ cell proliferation inhibitors (Th1) and CD4+RORγt+CD161+ (Th17) and significantly reduce their ability to produce pro-inflammatory cytokines such as IFN-γ, TNFα and IL-1β (Th1) and IL-17A and IL-22 (Th17) (). In fact, using CD4+CD45RO+CCR6+ positive cells (Th17 cells), it has been shown that human BM-MSCs induce the generation of Th17 cells with regulatory characteristics in an inflammatory environment characterized by a decrease in the RORC expression, an increase in the FoxP3 expression and the acquisition of immunosuppressive functions (). This was corroborated in a study with MSCs of human adipose tissue that were able to reduce the production of IL-17, TNF and IFN-γ and induce T cells of production IL-10 in vitro in T cells of specific peripheral blood of RA patients collagen (). It is well admitted that MSCs co-cultive with CD4+ purified T cells induce the expression of CD25High and FoxP3+ on the surface of these last T cells in a contact-dependent manner (, ). The generation of these CD4+CD25+FoxP3+ It has been shown that the treg is, in part, dependent on the ICOSL expression by MSCs (). In fact, ICOS is expressed in activated T memory cells, including Th17 cells, therefore through a MSCs contact cell mechanism it was proposed to interact with Th17 cells memory and generate Treg cells memory. In another study, it was reported that MSCs were able to recruit CD4+CD25+CD45RA+ and CD4+CD25+CD45RO+ treg cells, but the subpopulation of naive Treg cells was further recruited. In addition, MSC regulates and maintains the suppressive function of memory Bring cells over time (). Therefore, in the context of the RA, the regulation of the Treg memory cell by MSCs is critical as they are more plastic than the naive Treg cell population (). Together, these studies provide evidence that MSCs not only increase the generation of Treg cells and the production of IL-10 or TGFβ1 but also extend their immunosuppressive capacity while maintaining their phenotype (FoxP3+ CD127low) and functions (, ). This is a critical function performed by MSC, considering that the RA Treg of patients exhibits an altered functionality. In addition, MSCs suppressing the secretion of IL17-A by Th17-effector-memory cells decrease the acute or chronic activation of these cells in the RA. Thus, MSCs not only inhibit IL-17 production but also induce the reprogramming of Th17 immunopatogenic memory cells into T cells with regulatory phenotype and functions () (Summarized in ). MSCs dampen RA progression through the induction of the balance between Th17 memory and Treg cells. In RA, MSCs may decrease the frequency of the pathogenic memory Th17 cells and the production of pro-inflammatory cytokines such as IL-17, IL-22, and GM-CSF and promote their differentiation to an anti-inflammatory phenotype. In parallel, MSCs could also increase memory capacity Treg cells to produce anti-inflammatory cytokines such as IL-10 or TGFβ1 and prolong their immunosuppressive capacity while maintaining their anti-inflammatory phenotype. Future Perspective MSCs are multipotent cells with extensive immunomodulatory properties, therefore they have been proposed as a candidate for choice for the treatment of autoimmune diseases including the RA. However, the clinical benefit for the RA after 3 months of MSC administration has shown inconsistent positive effects. Therefore, it is necessary to increase the number of patients and studies in order to draw solid conclusions on the therapeutic effects of MSC in the RA. In addition, it is important to note that clinical trials with MSC were injected in patients with severe and refractory RA that suggest that the treatment of MSC could be more effective at early stages of the disease (). In addition, the studies only evaluated the short-term effectiveness of MSCs, from 3 to 8 months, and therefore it is still necessary to address the evaluation of MSC's long-term effectiveness. On the basis of the topics discussed here we believe that it is necessary to address other studies to evaluate the effect of MSC treatment on T pathogenic memory cells derived from RA patients. Since MSCs on migrating injection to the site of inflammation if you find a high number of T cells of proinflammatory memory it is essential to evaluate the effect of MSCs on T cells of RA memory that has not been explored. In addition, it is mandatory to achieve detailed immune surveillance of RA patients that analyzes the dynamics of PAD cells and not pathogenic memory on the infusion of CMS. ConclusionMemory T cells have been studied largely because of their fundamental role in the pathogenesis of autoimmune diseases such as the RA. Although the T-cell pro-inflammatory memory-exhibit harmful effect on the RA, its plasticity potential still offers an approach to explore to better control the progression of the RA. In this context, MSCs, powerful immunosuppressive cells that are capable of inhibiting proliferation and pro-inflammatory T cell functions while inducing the generation of T-regular cells, represent a strong candidate to choose for RA treatment. Thus, deciphering the base of the cross between MSCs and T-memory pathogenic cells in RA will pave the way to develop new and powerful strategies to successfully improve MSC-based therapies. Author ContributionsNL-C, RC-L, FD, RE-V and PL-C. wrote the manuscript with the input of MP-M, MT, SB, MW and FE. Conflict of interest The authors state that the investigation was conducted in the absence of commercial or financial relations that could be interpreted as a potential conflict of interest. FootnotesFunding. This work was supported by the National Fund for Scientific and Technological Development 408 (FONDECYT) Initiation 11160929, Inserm, the University of Montpellier and the Société Française de Rhumatologie (SFR). ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA

Wrist Rheumatoid Arthritis Treated with Stem Cell Therapy - Dr. Dennis Lox Stem Cell Therapy
Medical Facts of STEM CELL THERAPY in Rheumatoid Arthritis
Stem Cell Therapy For Rheumatoid Arthritis in 2021
Rheumatoid Arthritis (RA) | ANOVA IRM Germany
Affordable Stem Cell Therapy for Rheumatoid Arthritis in India | Tour2India4Health Blog

Stem Cell Therapy a Possible Treatment for Rheumatoid Arthritis

Rheumatoid Arthritis and Stem Cell Treatment
Stem Cell Therapy for Rheumatoid Arthritis | MedAdvisor | Stem Cell Therapy, Treatment Options, Patient Peer Groups

Get the Benefits of Stem Cell Therapy for Rheumatoid Arthritis in India – Stem Cell Therapy in India Blog

Stem Cell Therapy for Rheumatoid Arthritis - Stem Cell Institute

Medical Facts of STEM CELL THERAPY in Rheumatoid Arthritis
Stem Cell Therapy for Arthritis - Rheumatoid & Osteoarthritis

Stem Cell Treatment for Rheumatoid Arthritis in India - Stem Cell Therapy in India

Stem cell therapy for rheumatoid arthritis: What to know
Rheumatoid Arthritis (RA) | ANOVA IRM Germany

Stem Cell Therapy for Rheumatoid Arthritis - YouTube

Stem Cell Treatment Rheumatoid Arthritis in Delhi, India - Stem Cell Care India |

Rheumatoid Arthritis. Stem cell therapy and treatment for Rheumatoid Arthritis

Stem Cell Therapy Could Help Treat Rheumatoid Arthritis -
Stem Cell Therapy for Rheumatoid Arthritis | Innovations Medical

If you've had a stem cell treatment, how was your experience? | The Niche

Stem cell therapy for rheumatoid arthritis: What to know
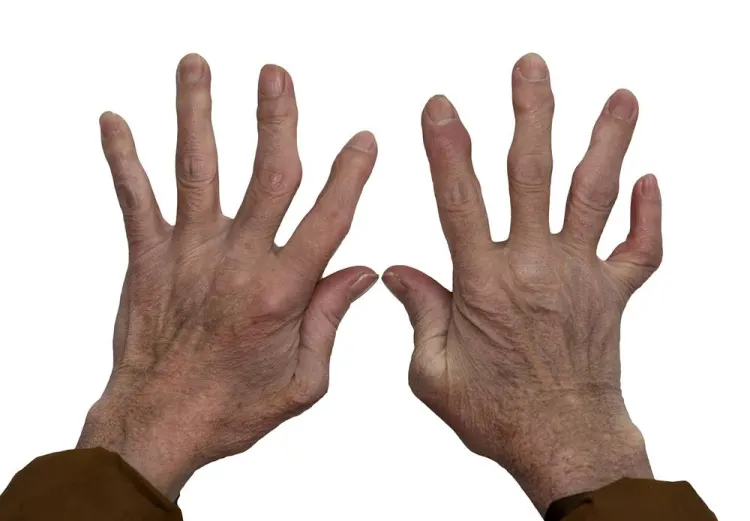
Stem Cell Therapy For Rheumatoid Arthritis in India - Affordable Stem Cell Treatment for Rheumatoid Arthritis| stemcelltherapyinindia.com

Stem Cell Therapy for Rheumatoid Arthritis by georginaewagne720 - issuu

Stem Cell Therapy & Treatment for Rheumatoid Arthritis

Stem Cells Therapy for Arthritis and Arthrosis: How it can be treated by Swiss Medica | Caerphilly Observer
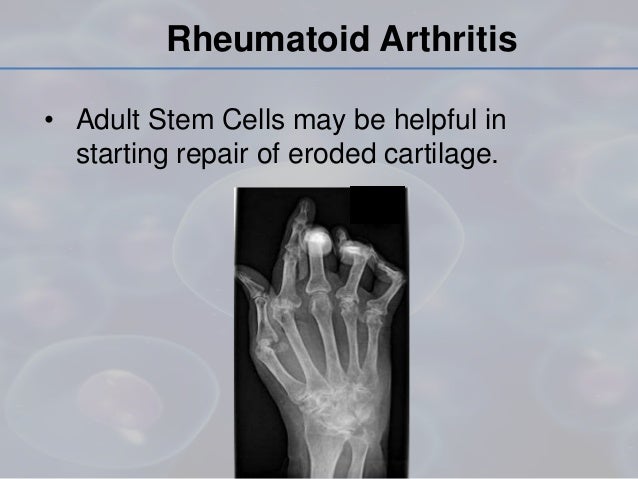
Stem cell therapy
Can Stem Cell Therapy be a Viable Rheumatoid Arthritis Treatment? – My Rheumatoid Arthritis Diary
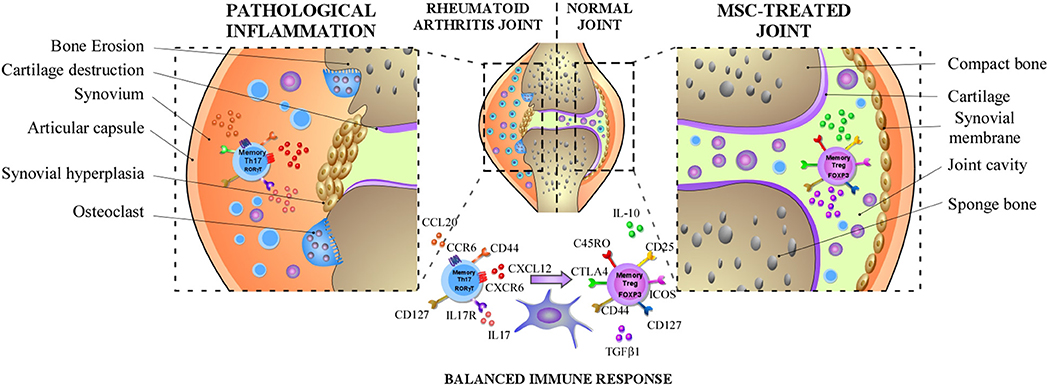
Frontiers | Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response | Immunology

Rheumatoid Arthritis Stem Cells Treatment, Autoimmune, Inflammation, Joints, Cartilage, Therapy Costa Rica

Stem Cell Therapy a Possible Treatment for Rheumatoid Arthritis
Stem Cell Therapy for Arthritis - Rheumatoid & Osteoarthritis
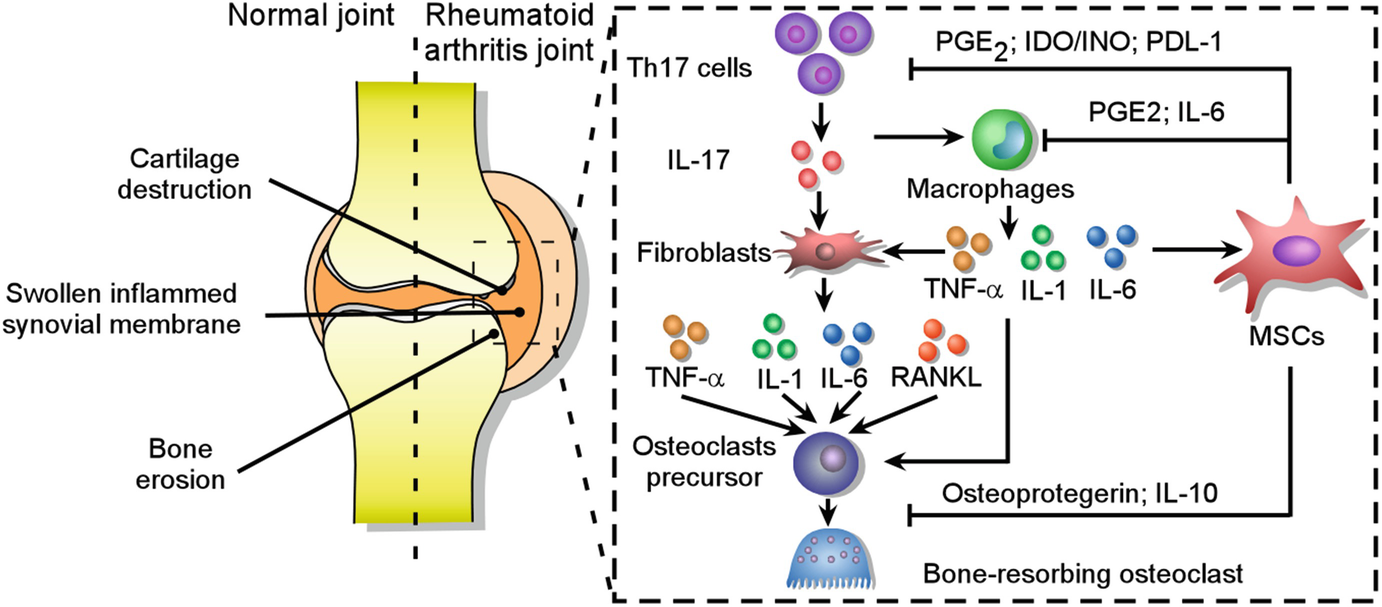
From Bench to Bedside of Mesenchymal Stem Cells Use for Rheumatoid Arthritis Treatment | SpringerLink

Stem Cell Therapy For Rheumatoid Arthritis in 2021
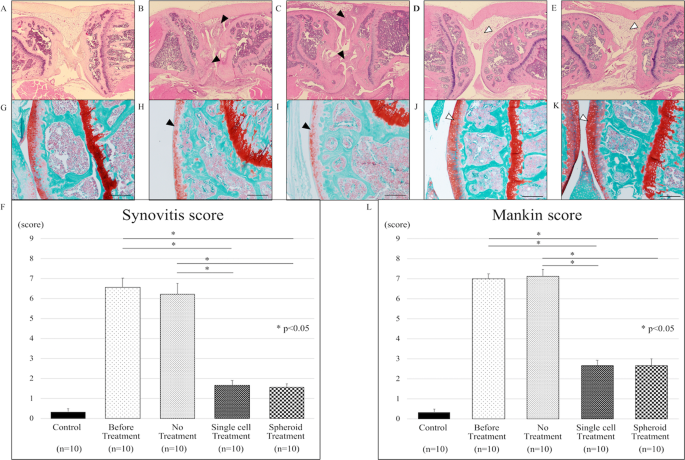
Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis | Scientific Reports

Stem Cell Therapy for Arthritis

Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis - EBioMedicine

New stem cell-based treatment for rheumatoid arthritis | Horizon 2020

Future of Stem Cell Therapy in Rheumatoid Arthritis Treatment Analyzed | Medgadget

Stem Cell Therapy for Rheumatoid Arthritis: Lynx Healthcare: Pain Management Practice
Posting Komentar untuk "stem cell treatment for rheumatoid arthritis"